Просмотр содержимого документа
«Каталитические свойства железосодержащих слоистых алюмосиликатов в фотоокислении красителя "метиловый зеленый"»

Buryat state University
Department of inorganic and organic chemistry
Shadrina Olesya Andreevna
THE CATALYTIC PROPERTIES OF F E -CONTAINING LAYERED ALUMINOSILICATE IN THE PHOTOOXIDATION OF THE DYE "METHYL GREEN"
Supervisor: doctor of chemical Sciences, associate professor Khankhasaeva S. Ts.
Ulan-Ude
2017
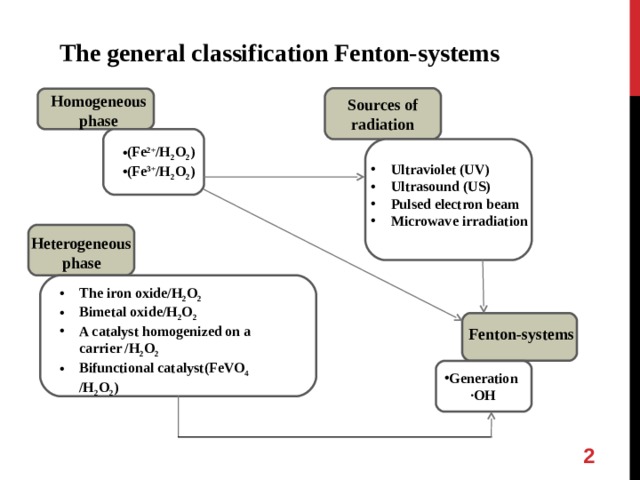
The general classification Fenton-systems
Homogeneous phase
Sources of radiation
- (Fe 2+ /H 2 O 2 )
- (Fe 3+ /H 2 O 2 )
- Ultraviolet (UV)
- Ultrasound (US)
- Pulsed electron beam
- Microwave irradiation
Heterogeneous phase
- The iron oxide/H 2 O 2
- Bimetal oxide/H 2 O 2
- A catalyst homogenized on a carrier /H 2 O 2
- Bifunctional catalyst(FeVO 4 /H 2 O 2 )
Fenton-systems
·OH
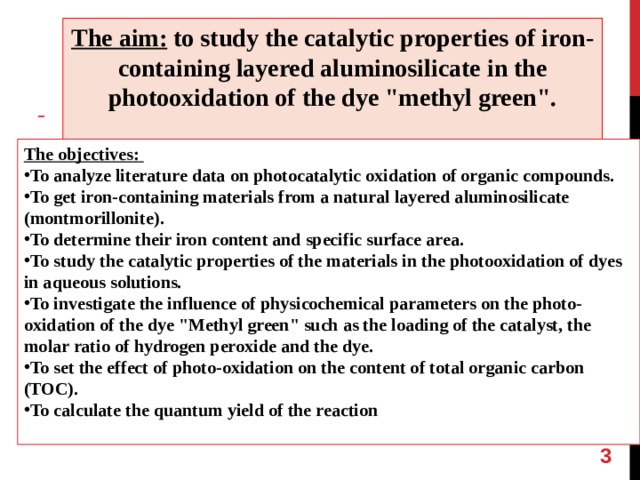
The aim: to study the catalytic properties of iron-containing layered aluminosilicate in the photooxidation of the dye "methyl green".
The objectives:
- To analyze literature data on photocatalytic oxidation of organic compounds.
- To get iron-containing materials from a natural layered aluminosilicate ( montmorillonite ) .
- To determine their iron content and specific surface area.
- To study the catalytic properties of the materials in the photooxidation of dyes in aqueous solutions.
- To investigate the influence of physicochemical parameters on the photo-oxidation of the dye "Methyl green" such as the loading of the catalyst, the molar ratio of hydrogen peroxide and the dye.
- To set the effect of photo-oxidation on the content of total organic carbon (TOC).
- To calculate the quantum yield of the reaction
The methods of research:
- Chemical methods of analysis
- UV-Vis spectrophotometry
- Low-temperature adsorption of nitrogen
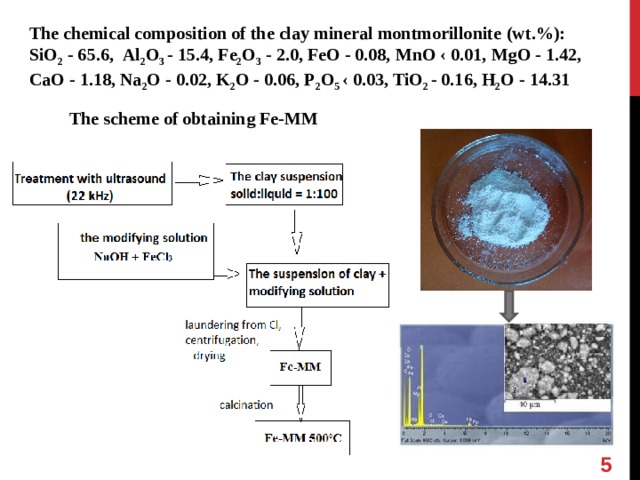
The chemical composition of the clay mineral montmorillonite ( wt .%):
SiO 2 - 65.6, Al 2 O 3 - 15.4, Fe 2 O 3 - 2.0, FeO - 0.08, MnO ‹ 0.01, MgO - 1.42, CaO - 1.18, Na 2 O - 0.02, K 2 O - 0.06, P 2 O 5 ‹ 0.03, TiO 2 - 0.16, Н 2 О - 14.31
The scheme of obtaining Fe -М М
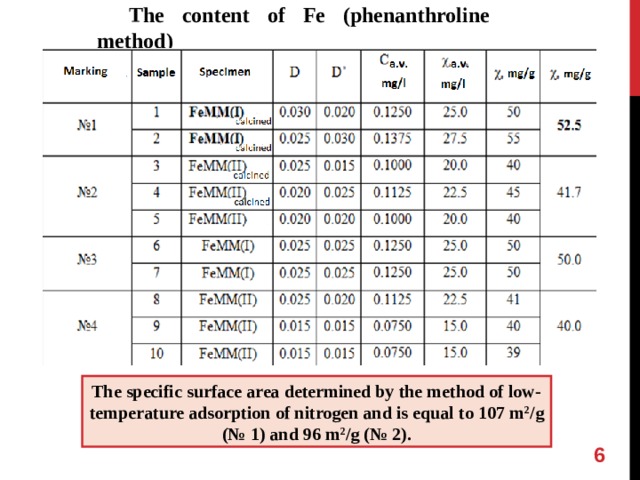
The content of Fe (phenanthroline method)
The specific surface area determined by the method of low-temperature adsorption of nitrogen and is equal to 107 m 2 /g ( № 1) and 96 m 2 /g ( № 2).
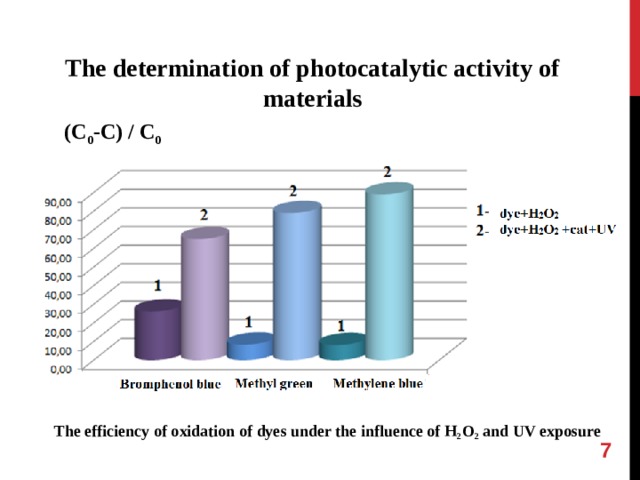
The determination of photocatalytic activity of materials
( С 0 -С ) / C 0
The efficiency of oxidation of dyes under the influence of H 2 O 2 and UV exposure
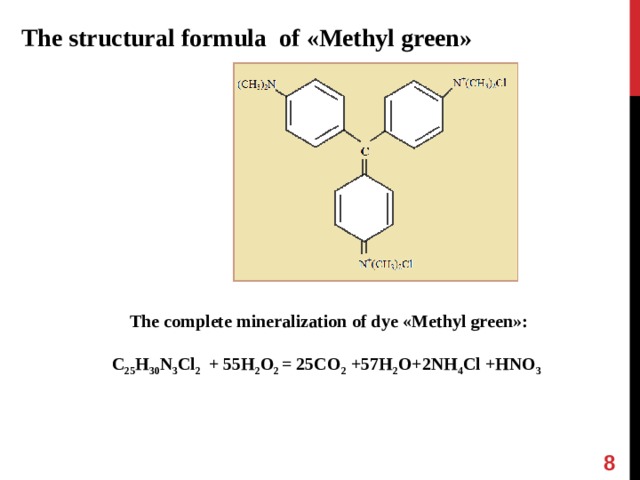
The structural formula of « Methyl green »
The complete mineralization of dye « Methyl green » :
C 25 H 30 N 3 Cl 2 + 55H 2 O 2 = 25CO 2 +57H 2 O+2NH 4 Cl +HNO 3
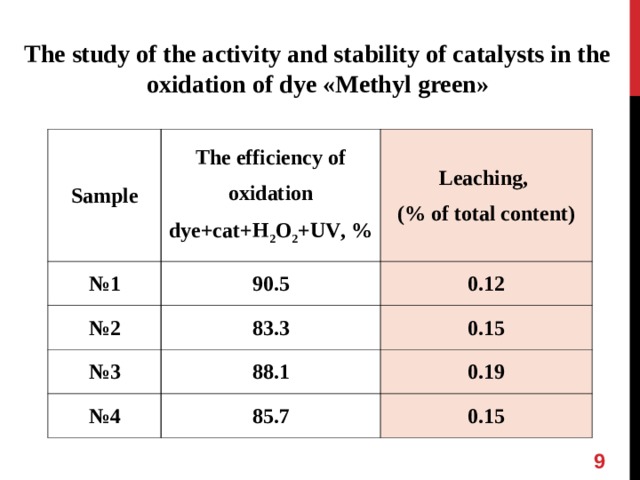
The study of the activity and stability of catalysts in the oxidation of dye « Methyl green »
Sample
The efficiency of oxidation
dye + cat + H 2 O 2 + UV , %
№ 1
Leaching,
(% of total content)
90.5
№ 2
83.3
0.12
№ 3
88.1
№ 4
0.15
85.7
0.19
0.15
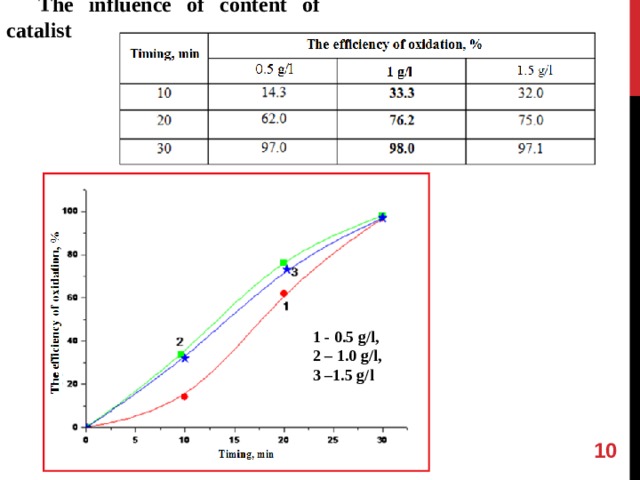
The influence of content of catalist
1 - 0.5 g / l ,
2 – 1.0 g / l ,
3 –1.5 g / l
![The dependence of the efficiency of oxidation from the molar ratio [H 2 O 2 /methyl green] 0.5 (1), 0.75 (2), 1.0 (3), 1.25 (4), 1.5 (5), 1.75 (6)](https://fsd.multiurok.ru/html/2020/10/29/s_5f9a4527187ea/img10.jpg)
The dependence of the efficiency of oxidation from the molar ratio [H 2 O 2 /methyl green]
0.5 (1),
0.75 (2),
1.0 (3),
1.25 (4),
1.5 (5),
1.75 (6)
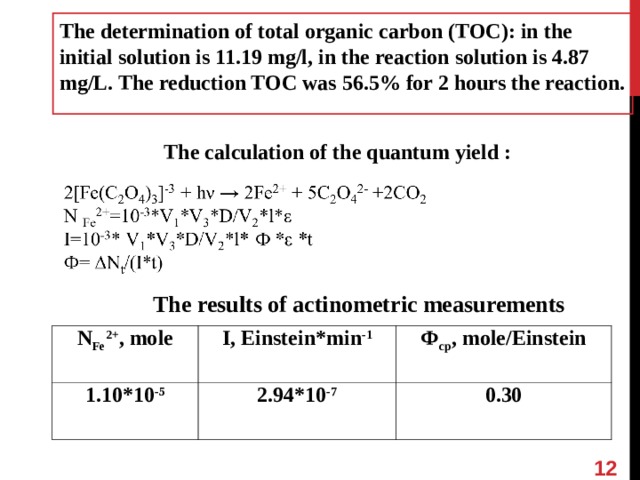
The determination of total organic carbon (TOC): in the initial solution is 11.19 mg/l, in the reaction solution is 4.87 mg/L. The reduction TOC was 56.5% for 2 hours the reaction.
The calculation of the quantum yield :
The results of actinometric measurements
N Fe 2+ , mole
1 . 1 0 *10 - 5
I , Einstein*min -1
Ф ср , mole/Einstein
2.94*10 -7
0.30
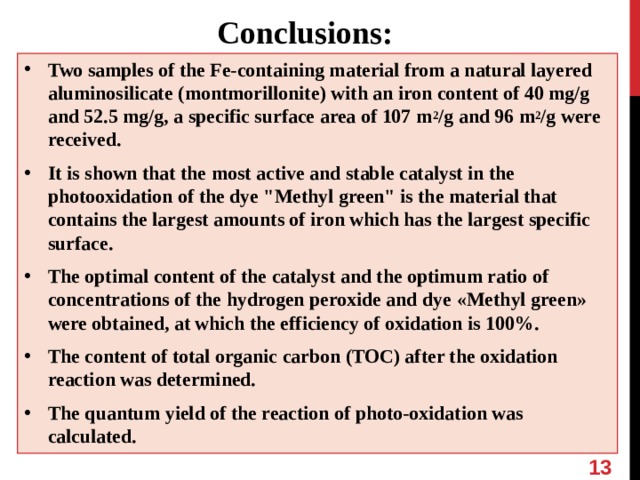
Conclusions:
- Two samples of the Fe-containing material from a natural layered aluminosilicate ( montmorillonite ) with an iron content of 40 mg/g and 52.5 mg/g, a specific surface area of 107 m 2 /g and 96 m 2 /g were received.
- It is shown that the most active and stable catalyst in the photooxidation of the dye "Methyl green" is the material that contains the largest amounts of iron which has the largest specific surface.
- The optimal content of the catalyst and the optimum ratio of concentrations of the hydrogen peroxide and dye « Methyl green » were obtained, at which the efficiency of oxidation is 100%.
- The content of total organic carbon (TOC) after the oxidation reaction was determined.
- The quantum yield of the reaction of photo-oxidation was calculated.

THANK YOU FOR ATTENTION
















![The dependence of the efficiency of oxidation from the molar ratio [H 2 O 2 /methyl green] 0.5 (1), 0.75 (2), 1.0 (3), 1.25 (4), 1.5 (5), 1.75 (6)](https://fsd.multiurok.ru/html/2020/10/29/s_5f9a4527187ea/img10.jpg)




















